"This really has the possibility of ending HIV transmission," said Greg Millett, public policy director at amfAR, The Foundation for AIDS Research."
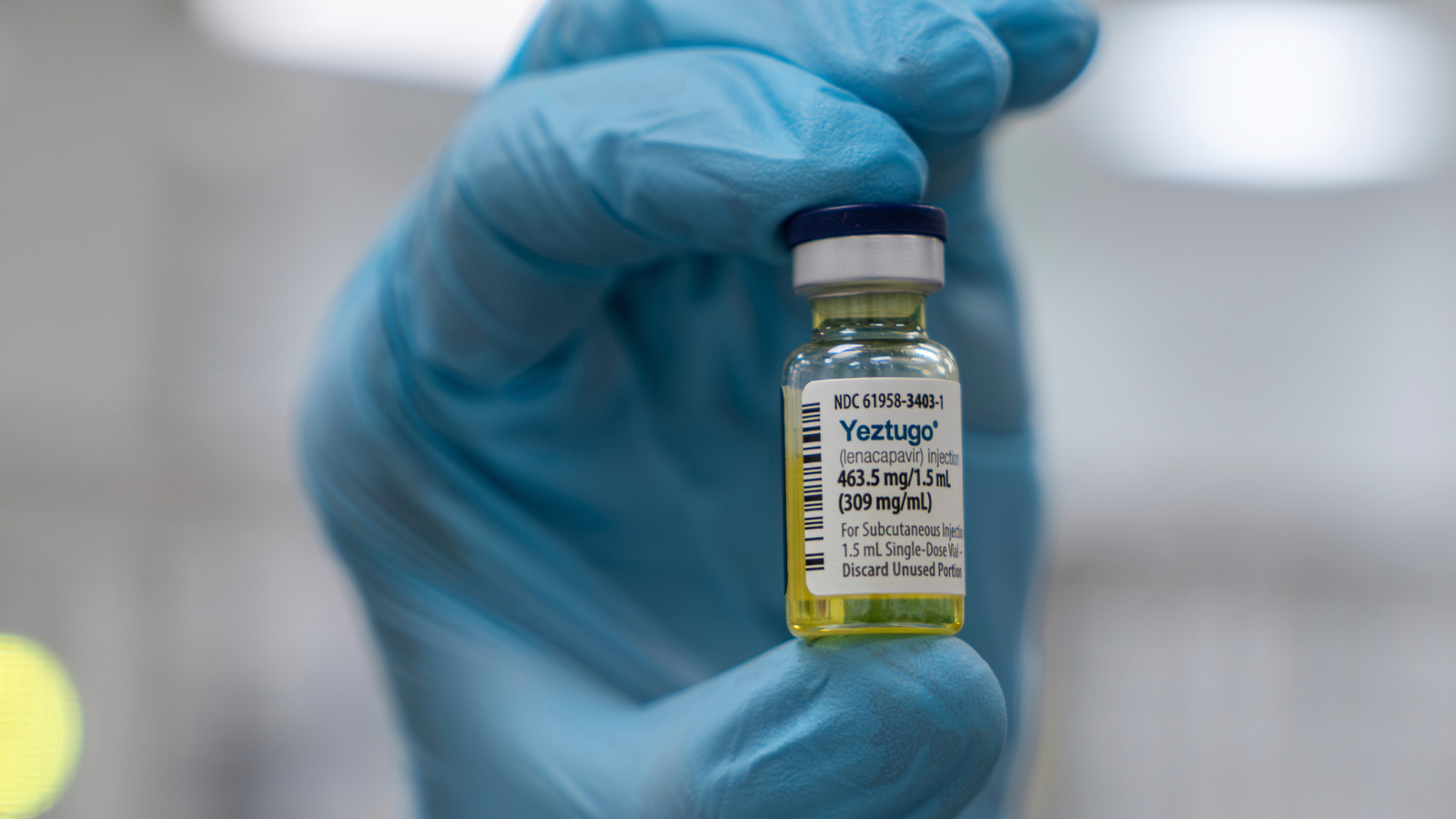
"Lenacapavir's six-month protection makes it the longest-lasting type, an option that could attract people wary of more frequent doctor visits or stigma from daily pills."
"Gaping holes in the system in the U.S. and globally are going to make it difficult for us to make sure we not only get lenacapavir into people's bodies but make sure they come back."
"While a vaccine to prevent HIV still is needed, some experts say the shot - a drug called lenacapvir - could be the next best thing."
Gilead Sciences announced the U.S. approval of lenacapvir, the world's first HIV preventive shot administered twice a year. Experts believe it could significantly impact HIV transmission rates, particularly among high-risk groups, by nearly eliminating new infections. With its longer-lasting effect compared to daily pills, it may appeal to individuals hesitant to take regular medication. However, systemic challenges like public health cuts and foreign aid reductions hamper access efforts. Despite this promise, experts stress the importance of addressing systemic issues to ensure implementation and return visits for treatment.
Read at ABC7 Los Angeles
Unable to calculate read time
Collection
[
|
...
]